Funding
Most researchers embarking on a repurposing project will be seeking funding at some point. Most of the mainstream translational funding schemes, such as MRC Developmental Pathway Funding Scheme and the LifeArc Philanthropic Fund, will welcome repurposing projects. There are other schemes dedicated solely to repurposing. Some of the major schemes are listed below.
MRC translational schemes and joint NIHR schemes
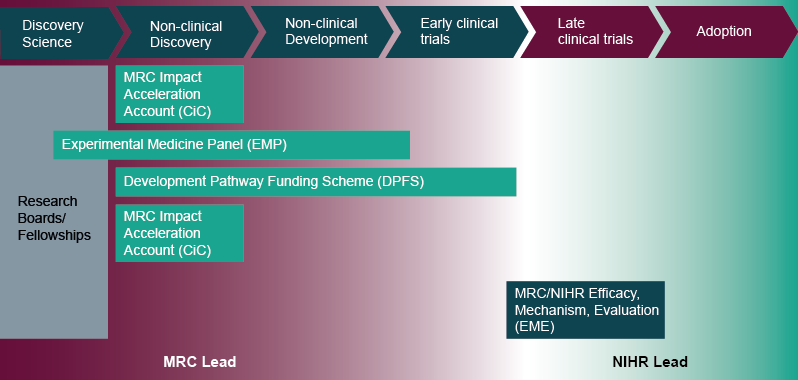
- MRC Impact Acceleration Accounts (formerly Confidence in Concept): funding devolved to research organisations to support preliminary work to establish the viability of an approach and de-risk projects across the whole translational pathway. This may include data gathering to support drug repurposing projects. Decision-making and administration is managed locally, usually through a university's Translational Research Office.
- MRC Experimental Medicine: supports projects with the primary objective of investigating the causes, progression and treatment of human disease, with a clear path to clinical impact. Projects must involve an experimental intervention or challenge in humans, which has been designed to validate a mechanistic hypothesis based round a gap in understanding of human pathophysiology. This may include the use of drugs with established safety profiles in new settings or conditions (for example, repurposing drugs as tool compounds to probe disease mechanism). Annual budget c. £10 million; two rounds per year.
- MRC Developmental Pathway Funding Scheme: The MRC's flagship translational scheme, supporting academically-led projects aiming to develop and test novel and repurposed therapeutics, medical devices, diagnostics and other interventions across all areas of unmet medical need. Repurposing projects can include pre-clinical or clinical studies up to and including phase IIa. Annual budget c.£30 million; three rounds per year.
- Mechanistic work in vitro or in animals, or development and validation of disease models, which may utilise repurposed compounds, would fit within the MRC Research Boards:
- MRC/NIHR Efficacy and Mechanism Evaluation: supports later phase (IIb and III) clinical trials aiming to test the efficacy of interventions, including repurposed drugs. This includes proof of clinical efficacy, size of effect, and long-term safety in a well-defined population. Where appropriate, the programme encourages hypothesis-testing mechanistic studies integrated within the main efficacy study. This scheme is available across the whole UK.
Other NIHR schemes
Medicines Repurposing Programme
The Medicines Repurposing Programme is a national multiagency initiative which identifies and progresses opportunities to use existing medicines in new ways, outside the current marketing authorisation. There is an open call for healthcare professionals, charities and companies to put forward candidate repurposing opportunities for consideration. Medicines prioritised to enter the programme receive a tailored package of support potentially including evidence generation, facilitating licence variation applications, and support for implementation.
The programme supports repurposing projects, in England, that have already achieved evidence of safety and efficacy in the target patient population, usually as part of a phase 2 trial but not always.
For eligibility and prioritisation criteria visit Medicines Repurposing Programme
The programme will carry out their own diligence re IP landscape/ treatment pathway etc when considering applications to the programme.
The process for application is explained on the scheme website
LifeArc Philanthropic Fund (rare diseases)
The LifeArc Philanthropic Fund provides grants and funding to academic researchers working to advance new treatments and diagnostics for rare diseases.
Charities also frequently offer support for repurposing projects in their particular disease area. Funders and charities are also open to co-funding such projects.
Depending on the IP situation around the therapy that you are looking to repurpose, the patent holder or, if off patent, the originator company, may also invest in a repurposing programme.

